Solid Oxide Fuel Cells
- Main Activities
- Proton Conducting Ceramics for SOFCs
- Introduction
- Approach, Facility and Capabilities
- Measurement and Analysis of SOFC Anode Gas Flow and Tortuosity
- Introduction
- Objectives
- Approach
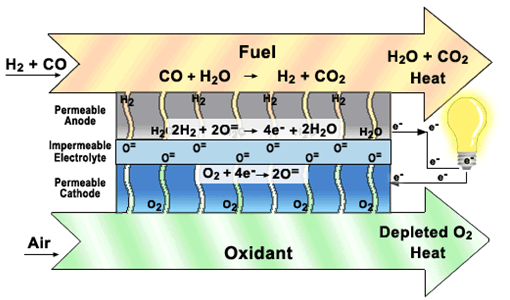
Oxide-ion conducting electrolyte based SOFC, Courtesy of SECA.
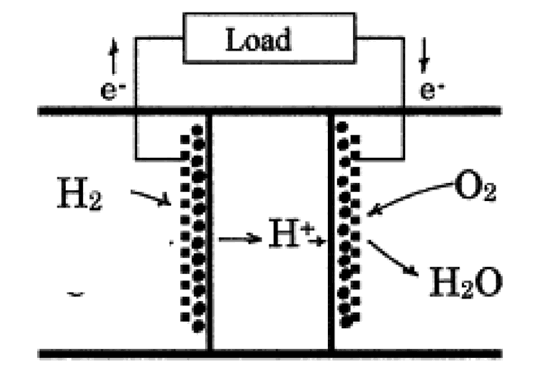
Proton conducting electrolyte based SOFC
Main Activites
- Synthesis, processing, and characterization of proton conducting ceramics for developing proton conducting electrolyte based SOFCs.
- Measurement and analysis of SOFC anode gas flow and tortuosity for various anode structures.
- Collaborative work with other groups, such as electrochemical impedance spectroscopy measurement and analysis, dual-atmosphere high temperature testing of SOFC materials and cells fabricated by us and other groups.
- Initial works on SOFCs with liquid metal anodes.
Proton Conducting Ceramics for SOFCs
Introduction
Compared with conventional oxide-ion conducting electrolyte based SOFCs, proton conducting electrolyte based SOFCs have several advantages. For example, the potential to lower SOFC operation temperature to 500-700C, thereby significantly lowering the cost of SOFCs. Also, no water vapor is produced in the anode side of proton conducting SOFCs, therefore avoiding dilution of the fuel and improving fuel efficiency. The present work is to develop dense and chemically stable proton conducting ceramics with high proton conductivity, for the application of intermediate-temperature SOFCs. Typical proton conducting ceramics studied are doped BaCeO3 and doped BaZrO3.
Our SOFCs with proton-conducting electrolyte have high power density above 1 W/cm2 and run well in both the fuel cell and electrolysis modes. The electrolyte material is also tested for use in hydrogen separation membranes, using our gas chromatograph apparatus.
Approach, Facility and Capabilities
- Nanometer ceramic powders are synthesized by the Glycine-Nitrate process.
- Bulk ceramics are fabricated by pressing and sintering processes.
- Ceramic membranes (i.e., thick films) are fabricated by screen printing and tape casting processes.
- Proton conductivities of the samples at different temperatures (20-1000C) and various H2, O2, and water vapor atmospheres are measured using impedance spectroscopy techniques (a Solartron 1260 and 1287 electrochemical measurement system with a high temperature testing set-up).
- Fuel cell testing and H2, O2, and water vapor concentration cell testing are performed in a dual-atmosphere high temperature testing system (20-1000C).
- DC and AC electrochemical properties of the materials and cells are measured by a Solartron 1260 and 1287 electrochemical measurement system.
- Microstructures and compositions of the samples are characterized using the techniques in the Image and Chemical Analysis Lab(ICAL) in our department, such as XRD, FE-SEM, EDS, XPS, SIMS, Auger, and AFM.
Measurement and Analysis of SOFC Anode Gas Flow and Tortuosity
Introduction
Anode-supported solid oxide fuel cells require careful design of the relatively thick anode, so that the fuel gas flow through the anode to the solid electrolyte and the electrochemical reaction by-product gas flow out of the anode are not excessively impeded. Currently, the factors that control concentration polarization at the anode are not completely understood. A comprehensive understanding of these diffusion limitations in binary and ternary gas systems will ultimately allow the design of more effective microstructurally engineered electrodes in SOFCs, steam electrolyzers, gas separation membranes, and other high-temperature electrochemical systems.
One key variable that is essential in the understanding of gas diffusion is the path length of the pores, referred to as the tortuosity, which is a measure of the actual path divided by the straight line path through the electrode. Given the complex three dimensional pore structure of fuel electrodes, the tortuosity is a difficult value to measure. Proper determination of tortuosity will allow the means for which concentration polarization can be accurately predicated. The present work performs an integrated experimental and theoretical approach to measure and analyze SOFC anode gas flow and tortuosity for various anode structures.
Objectives
- Determine the real tortuosity of electrode structures in SOFCs.
- Measure and analyze gas flow and counter diffusion in porous electrode layers.
- Elucidate the mechanisms for concentration polarization in SOFC anodes under binary and ternary flow.
- Develop new and improved analytical tools to predict concentration polarization for engineered microstructures and electrode designs.
- Validate polarization predictions with SOFC fuel utilization studies.
Approach
- The Stefan-Maxwell equation provides a set of differential equations for analyzing flows of each of several gas constituents. Under the condition of fuel gas starvation at the anode-electrolyte interface, these equations can be solved for the anode pore average tortuosity if other experimental parameters are known. Detailed analysis of the flow of the fuel gas, and the exhaust gas generated at the anode-electrolyte interface, has been initiated.
- A dual opposing flow test apparatus is constructed to determine actual diffusivities of counter flowing gases from which tortuosity can be estimated.
- SOFCs are also fabricated for fuel utilization studies to validate the theoretical and experimental results of this study.

