RBS
In Rutherford Backscattering (RBS), monoenergetic (~MeV) alpha particles (He nuclei) collide with target nuclei and are scattered by the Coulomb interaction. By studying the kinematics of this collision (well represented by a billiard ball model), the identity of target atoms can be uncovered. A detector is placed at a large angle (~170 degrees) with respect to the incident beam, and records: number of backscattered alpha particles vs. energy.
This technique is particularly useful for thin film analysis. RBS can yield information about 1) chemical composition, 2) atomic percentages, 3) film thickness, and 4) crystal structure.
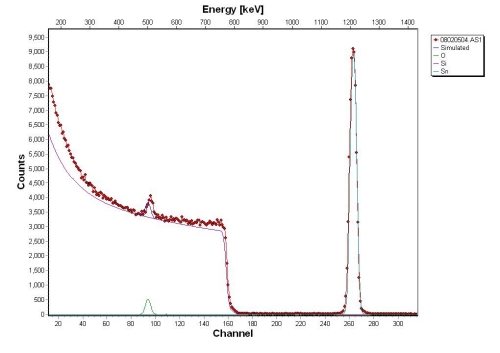
Above is an RBS spectrum of SnO2 on a Si substrate (higher channel # = higher energy). From right to left, the first peak we encounter is the tin signature. It occurs at the highest backscattered energy because it is the most massive element present in the sample. The next “peak” we see is that of the silicon, the second most massive element in the sample. Then we come across the oxygen peak. The tin and oxygen peaks are very narrow because the film is very thin (~25nm in this case). The silicon signature continues effectively to zero energy because the substrate is very thick (the ? particles penetrate ~1 micron into the sample). The deeper into the substrate the ? particles penetrate, the more energy they lose, thus the long tail on the silicon. This is how thin film thickness measurements can be made. The tin intensity is much greater than oxygen (although there are 2 oxygen atoms per tin atom) because there is a Z2 dependence in the scattering cross section. By knowing an element’s cross section for RBS, and using the relative intensities from an RBS spectrum, the atomic percentage of a sample can be calculated.
The above RBS spectrum was obtained in Dick Smith’s lab:
www.physics.montana.edu/ionbeams/ionbeams.html
